Preoperative Planning Tool Has the Potential for Changing the Management of Cerebral Aneurysm Treatment

SCOTTSDALE, AZ – November 8, 2017 – EndoVantage, LLC, a pioneer in cloud-based medical simulation technology, today announced its receipt of 510(k) clearance from the U.S. Food and Drug Administration for SurgicalPreview™, its preoperative planning system for the endovascular treatment of cerebral aneurysms.
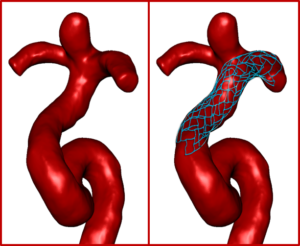
Using Advanced Analytics, SurgicalPreview™ provides insights into wall apposition, vessel shape and size, and foreshortening and can help a clinician evaluate the specific characteristics of device placement. (Image courtesy of EndoVantage, LLC)
A cerebral aneurysm is a weak spot in a blood vessel in the brain that balloons out and fills with blood. Aneurysms may burst and bleed into the brain, causing serious complications, including hemorrhagic stroke, permanent nerve damage, or death. Treatment includes the use of a device to divert blood flow away from the aneurysm. It can be challenging to select the precise size of device for a patient’s artery and to place it correctly. SurgicalPreview™ enables the clinician to plan the procedure before surgery and visualize how the device fits.
Using any computer with internet access clinicians can access SurgicalPreview™ and upload a patient’s CT imaging data. The system then converts that 2-dimensional data to a functional 3-dimensional model, enabling the clinician to manipulate the model as well as take anatomical measurements.
The clinician may also request computational models of multiple treatment scenarios for a patient, each representing a different treatment device and/or deployment strategy. SurgicalPreview™ includes a library of 3D models of neurological treatment devices approved by the FDA from which the clinician can select. The clinician can then view side- by-side static models and video models of the catheter delivery, landing and deployment. SurgicalPreview™ enables the clinician to evaluate multiple devices, sizes, placements, and deployment strategies.
“EndoVantage has put forth groundbreaking technology that will allow physicians to model and test a multitude of endovascular devices in patient specific scenarios without any risk to the patients themselves,” stated Felipe C. Albuquerque, MD, FAANS, Editor- In-Chief of the Journal of NeuroInterventional Surgery and Director of Endovascular Neurosurgery at Barrow Neurological Institute. “This revolutionary technology will facilitate the performance of complex, life-saving treatments. This is truly a new era in endovascular neurosurgery!” said Dr. Albuquerque.
“EndoVantage provides surgeons and interventionalists with a practical tool to “do the operation before the operation,” according to Bernard Bendok, MD, Chair, Department of Neurologic Surgery, Mayo Clinic Arizona. “This breakthrough technology promises to revolutionize precision surgery by better matching devices to patient specific anatomy. This important advance promises to substantially improve clinical outcomes and reduce risk of procedures. We are approaching a time when preoperative procedural modeling will be considered the standard of care. The tools which have been pioneered byEndoVantage will accelerate the advent of this next new exciting and higher standard in
healthcare,” stated Dr. Bendok..
In addition to preoperative planning, EndoVantage has been advancing the product development efforts of leading medical device companies using its simulation technology, revolutionizing the way devices are designed and developed. Its computational models provide far more detail than traditional physical testing and dramatically shorten development time. EndoVantage also tests devices in hundreds of patient anatomies without the patients, creating a “virtual clinical trial.”
This dramatically reduces the time and cost to regulatory approval and market.
About EndoVantage, LLC
EndoVantage, LLC develops and provides medical simulation services based on technology initially developed at Mayo Clinic and Arizona State University. Initial efforts are for endovascular procedures. For more information visit www.endovantage.com.
Media Contact:
Knox Witcher witcher@pinkstongroup.com
703.994.4943
