Flagship Biosciences, a fast growing pharmaceutical services company based in Flagstaff, AZ, is a leading example of the power of the collaborative nature of the Arizona bioindustry.
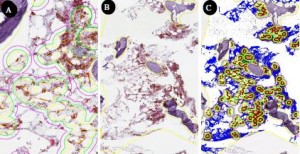
- In a collaboration between Flagship Biosciences and Dr. Mohamed Salama’s team at the University of Utah, Flagship’s computer algorithms can measure the number of myeloma cells within a given proximity of blood vessels in bone marrow samples. (Image courtesy of Flagship Biosciences)
The development of a drug can take over ten years, and cost over a billion dollars. One of the key components to evaluating toxicity and efficacy can only be observed by board-certified pathologists, evaluating tissue for response under a microscope. The pharmaceutical industry utilizes over 90 million glass slides a year, and the medical device firms add another 10 million slides worldwide. Pathology has been one of the last areas of clinical practice to embrace a fully digital workflow, due partly to the lack of acceptable slide scanning technology, and the order of magnitude higher resolution of the images over radiology.
Arizona is no stranger to the trend to digitize slides, with Dr. Ronald Weinstein from the University of Arizona as an early pioneer of telepathology and a leader in the field. Ventana Medical in Tucson is one of the largest players in immunohistochemistry, the preparation of the tissue and the creation of the glass slides prior to digitization, a business valued at $3.4 billion when acquired by Roche several years ago.
While the technology is improving rapidly, there was no services organization that was distinctly leveraging the technology in drug and device development.
Flagship was founded in 2009 by two veteran pharmaceutical pathologists from OSI and Novartis Pharmaceuticals, who saw the unique opportunity afforded by redesigning the entire tissue business around a digital workflow. Steve Potts, a scientist from Arizona, joined the company as CEO in 2010, and two top image analysis experts joined as well. One of these experts, Dr. Holger Lange, has written five of the last seven FDA clearances across the entire global digital pathology industry. Steve Potts’ team wrote the standards for the nacent industry for digital pathology compliance with 21 CFR 11 previously.
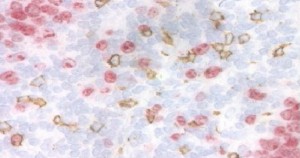
- Flagship Biosciences recently acquired IHCtech, a Colorado immunohistochemistry and histology firm with worldclass expertise in dual staining. Shown here is an example of two stains, one in brown to identify a given cell type (CD8 positive cells) and one in red to identify proliferating cells (Ki67). (Image courtesy of Flagship Biosciences)
“We have been profitable since we opened our doors, and were self-funded by working closely with customers from the very beginning. We have seen a 400% growth in business from 2010 to 2011, and are now working with over sixty biotech, pharma, and medical device clients” said Dr. Steve Potts. “We are adding additional tissue oriented services in order to provide a complete workflow to our clients, from tissue procurement, through immunohistochemistry to digital pathology and image analysis.”
The unique nature of a digital workflow allows Flagship to have pathology collaborations around the world, regardless of where the pathologist actually lives. “For a lymphoma drug study, we work with a top lymphoma pathologist in Southern California, for a myeloma study, a top expert in Utah, for prostate an academic medical center in Minnesota”. This collaboration allows the specialty pathologist to work closely with Flagship’s image analysis scientists in developing quantitative approaches that correlate well with what is observed by a pathologist visually on a slide.
Flagship has filed a number of patents to protect their unique image analysis approaches, and authored over seven peer-reviewed science papers in 2011.
While most of Flagship’s customers are at biotech and pharma locations around the world, there has been some strong collaborations formed with Arizona biotech firms. “The collaboration spirit in Arizona is remarkable” said Steve Potts. “the amount of additional business that Arizona groups like TGEN generate for other local biotech providers is astonishing. It has been an honor to work with Steve Gately’s TGEN Drug Development group (TD2), with a number of completed projects where TD2 applies their unique oncology expertise and Flagship adds our quantitative pathology.”
Arizona has a number of worldclass specialty contract research organizations, including TD2 in Oncology, Flagship Biosciences in pathology, Imaging Endpoints in radiology, Medelis in oncology and dermatology clinical trials, and SDC in statistics. The need for best-in-class specialty service is growing in the pharmaceutical industry, and Arizona with a skilled workforce, strong collaborative spirit, and industry friendly environment is positioned to lead in this area.
_______________
