The FDA approved the first biosimilar in the U.S. in March of 2015 with more expected to be approved in 2016. Now, on a state by state basis, the laws that govern how these new therapies can be dispensed are being updated. Arizona has the opportunity to do so in 2016 with HB 2310 sponsored by Dr. Regina Cobb, Vice Chairman of the House Health Committee.
HB 2310 – biological products; prescription orders passed the House Health Committee in a vote of 5 for, 0 against and 1 absent on Tuesday January 26, 2015. (Click here to watch the video.)
The Bill now moves to the House Rules committee for review before proceeding to the full House for a vote. Upon passage by the House, HB 2310 would move to the Senate for their review and consideration.
HB 2310 has strong support in both the House and the Senate on both sides of the aisle and has the following sponsors and co-sponsors:
HB 2310 Sponsors
| SPONSORS: | COBB | P | COLEMAN | P | MEZA | P |
| YEE | P | BORRELLI | C | BOYER | C | |
| BROPHY MCGEE | C | CARTER | C | CLARK | C | |
| FINCHEM | C | FRIESE | C | LAWRENCE | C | |
| MEYER | C | SHOPE | C | WENINGER | C | |
| BEGAY | C | DIAL | C | HOBBS | C | |
| SHOOTER | C |
| TITLE: | biological products; prescription orders |
Key FAQ’s
Question: What are “Biosimilars”
As part of the Affordable Care Act, the Federal Government created two new definitions of drugs “Biosimilars” and “Interchangeable Biologics”.
The term biosimilar refers to products that are marketed after expiration of patents, which are claimed to have similar properties to existing biologic products. Due to the complexity of biologics (they are manufactured using living organisms as compared to traditional pharmaceuticals which are chemical compounds that are combined) a biologic product can only be made that is similar, but not identical as generic drugs are.
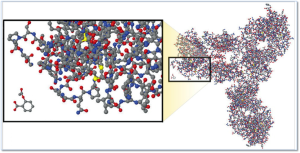
The image at right illustrates the comparative complexity of the most popular small molecule drug (aspirin) which is the small moleucar structure in the lower left of the image and a monoclonal antibody (large molecule /biologic product).
It will be up to the FDA to determine if a product is a biosimilar or if a product is so close to the original biologic that it is an interchangeable biologic. The FDA approved the first biosimilar in the U.S. in March of 2015 with more expected to be approved in 2016. Currently no interchangeable biologics have been approved by the FDA.
While the Federal government may review, approve and designate the class of these products, the states will each determine the process for substitution through each state’s Pharmacy Act.
Arizona will need to update its Pharmacy Act to define classes of biosimilars and establish the process for substitution for interchangeable biologics. Pharmacists in Arizona will not be authorized to substitute interchangeable biologocs (and save both patients and payers money) without the consent/permission of the prescriber until we update our Pharmacy Act.
Question: What will an update to Arizona’s Pharmacy Act will need to address
An update to the Pharmacy Act would need to establish the process of how these interchangeable biologics may be automatically substituted by pharmacists AFTER the FDA has approved them as interchangeable biologics and the products are available on the market. If we do not have a process in Arizona, there can be no substitution here without it being approved by the prescribing medical professional. This extra step, asking the physician for consent/permission, will result in more work for the prescribers and the pharmacists as well as delays for the patient or the patient’s caregiver.
These changes would NOT …
- limit access by prescribing medical professionals. The day these drugs are available, they can be prescribed for patients.
- require intervention by the prescribing medical professional before a substitution is made.
- indicate that one product is better or worse than the other.
- require a substitution to occur.
- make any changes to how we handle the automatic substitution of generic drugs
- require ANY state or federal funding
Key aspects of the Arizona biosimilars bill include:
- Only an interchangeable biologic can be substituted.
- Physicians retain the right to use “Dispense As Written”.
- Patients are notified of the substitution.
- Pharmacy record retention to match the generic statute.
- A communication process of what product was dispensed for a complete patient medical record.
Why is communication with the prescriber so important?
Biologics are advanced prescription drugs to treat cancer, rheumatoid arthritis, Crohn’s disease, psoriasis, and other debilitating diseases. Collaboration and clear communication among physicians, pharmacists, and patients is essential to good patient care and is especially important given the seriousness of the conditions being treated with biologic medications. In order to avoid confusion, both physician and pharmacist must know exactly which biologic medicine is dispensed to the patient.
It is important for both patients and payers to have the benefit of the pharmacist being able to substitute the potentially less expensive interchangeable biologics as soon as they are available. The practice of medicine and patient care is an art of managing many variables. A slight variation can result in a patient responding better or less favorably to any method of care. Even the closest products, the interchangeable biologics, will have some slight variations and could react differently with individual patients. For this reason it is critically important that information flow back to the prescribing medical professional and into the patient record whenever one of these interchangeable biologic products are substituted.
A 2012 ASBM survey of U.S. physicians found that 88% considered notification of the physician in the event of a substitution “very important or critical” (Source)
Key Definitions: Since these are not terms that all of us use every day, the FDA provided a simple FAQ for consumers to clarify the definitions. The FDA will also maintain a reference for healthcare providers in the Purple Book which provides lists of licensed biological products with reference product exclusivity and biosimilarity or interchangeability Evaluations. The FDA maintains a similar reference for drugs called the Orange Book which is used extensively today.

Organizations in SUPPORT of HB 2310
Biological Products; Prescription Orders
Pharmacists Substitute Interchangeable Biologics
and Communicate Substitution to Prescriber
- AbbVie
- Allergan
- American Cancer Society Cancer Action Network
- Amgen
- Arizona Myeloma Network
- Arizona Osteopathic Medical Association
- Arthritis Foundation
- Astellas
- AZBio
- BIO
- Biogen
- Boehringer Ingelheim
- Bristol Myers Squibb
- Coherus
- EMD Serono
- Express Scripts
- Genentech
 GlaxoSmithKline
GlaxoSmithKline- GPhA
- Horizon Pharma
- ICAN, International Cancer Advocacy Network
- Johnson & Johnson
- Lilly
- Merck
- Novo Nordisk
- Pfizer
- PhRMA
- Sandoz/Novartis
- Sanofi
- Southwest Chapter, Crohn’s & Colitis Foundation
- Takeda
- Teva
- UCB