Demonstrates Growing Acceptance As The New Standard Of Care For End-Stage Biventricular Heart Failure
“The fact that these top U.S. transplant centers offer the SynCardia Total Artificial Heart demonstrates its growing acceptance as the new standard of care for end-stage biventricular heart failure,” said Michael Garippa, SynCardia CEO and President.
In June, U.S. News and World Report published its annual list of “Best Children’s Hospitals” for cardiology and heart surgery. Among the top 10 were seven pediatric centers that offer the SynCardia Total Artificial Heart: #2 Children’s Hospital of Philadelphia (CHOP), #3 Texas Children’s Hospital, #5 Cincinnati Children’s Hospital Medical Center, #11 Children’s Hospital of Wisconsin, #26 Mattel Children’s Hospital UCLA, #32 Shands Hospital for Children at the University of Florida and #34 Phoenix Children’s Hospital.
As of July 18, SynCardia Certified Centers worldwide have performed a record-breaking 100 implants of the SynCardia Total Artificial Heart in 2013. This milestone was achieved three months earlier than in 2012, when the 100th implant was performed on Oct. 16. Worldwide, there have been more than 1,200 implants of the Total Artificial Heart, accounting for more than 315 patient years of life.
There are currently 87 SynCardia Certified Centers worldwide, including 48 in the U.S. An additional 33 centers are currently completing SynCardia’s four-phase certification program.
About the SynCardia temporary Total Artificial Heart
SynCardia Systems, Inc. (Tucson, AZ) is the privately-held manufacturer of the world’s first and only FDA, Health Canada and CE approved Total Artificial Heart. Originally used as a permanent replacement heart, the SynCardia Total Artificial Heart is currently approved as a bridge to transplant for people suffering from end-stage heart failure affecting both sides of the heart (biventricular failure). There have been more than 1,200 implants of the Total Artificial Heart, accounting for more than 315 patient years of life on the device.
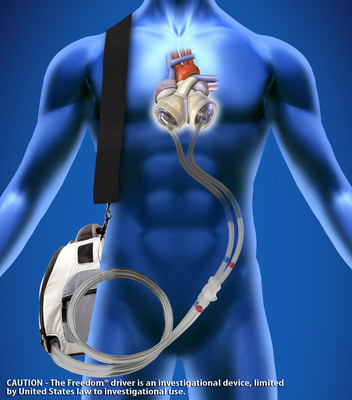
Similar to a heart transplant, the SynCardia Total Artificial Heart replaces both failing heart ventricles and the four heart valves. Unlike a donor heart, the Total Artificial Heart is immediately available at SynCardia Certified Centers. It is the only device that eliminates the symptoms and source of end-stage biventricular failure.
The Total Artificial Heart provides immediate, safe blood flow of up to 9.5 liters per minute through each ventricle. This high volume of blood flow helps speed the recovery of vital organs, helping make the patient a better transplant candidate.
Forbes Ranks SynCardia #69 Among “America’s Most Promising Companies”
In its February 2013 issue, Forbes selected SynCardia as one of “America’s Most Promising Companies” for the second consecutive year. On the list of 100 privately held, high-growth companies with bright futures, SynCardia was selected #69, moving up eight spots from its #77 ranking last year. See the full list of SynCardia Awards & Recognition here.
For up-to-date information, please visit: http://www.syncardia.com
SOURCE: SynCardia Systems, Inc.
Photo Credit: The SynCardia temporary Total Artificial Heart powered by the Freedom(R) portable driver is becoming the new standard of care for end-stage heart failure affecting both sides of the heart (biventricular failure). (Photo Courtesy of SynCardia Systems, Inc.) http://www.syncardia.com
