On February 3, 2026, the Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act was signed into law, establishing Medicare coverage for blood-based Multi-Cancer Early Detection (MCED) tests.

The Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act was includes as part of H.R. 7148, the “Consolidated Appropriations Act, 2026,” which makes further makes consolidated appropriations for the fiscal year ending September 30, 2026, and for other purposes and was signed by President Trump on February 3, 2026.
Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act
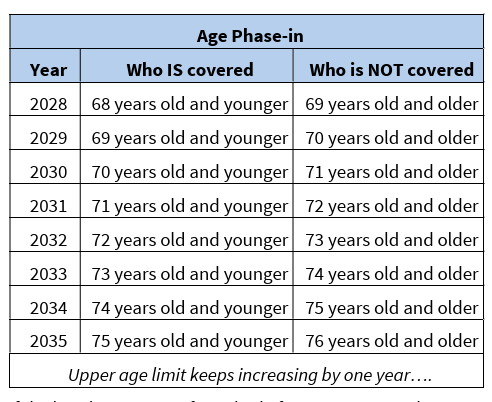
This bill allows, beginning in 2028, for Medicare coverage and payment for multi-cancer early detection screening tests that are approved by the Food and Drug Administration and that are used to screen for cancer across many cancer types, if the Centers for Medicare & Medicaid Services determines such coverage is appropriate. Coverage is limited to those under a certain age (age 68 in 2028, increased by one year every year thereafter) and to one test every 11 months. (Source – HR 842 Bill Summary: Congress.gov)
The Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act
The Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act will increase seniors’ timely access to multi-cancer early detection technology by creating a pathway to Medicare coverage once Multi-Cancer Early Detection (MCED) screening tests are approved (or authorized under 510K) by the FDA.
The new law allows for Medicare coverage of FDA approved/authorized MCED screenings.
As of this writing, no MCED screening tests have been FDA approved/authorized. A number of MCED screening tests are currently available as Laboratory Developed Tests (LDTs)
MCED screening tests do not replace existing screening tests but are intended to complement them/
Specifically, the new law
- Creates the authority for CMS to cover blood-based MCED tests and future test methods once approved by the FDA and shown to have clinical benefit.
- Maintains CMS’ authority to use an evidence-based process to determine coverage parameters for these new tests.
- Clarifies that (1) these new tools will complement, not replace, existing screenings and coverage and (2) cost sharing will not be impacted.
Bipartisan Support
In the Senate, S. 339 was sponsored by Senator Mike Crapo of Rhode Island with 67 co-sponsors including Arizona’s Senator Mark Kelly and Senator Ruben Gallego.
In the House, HR 842 was sponsored by Representative Jodey Arrington of Texas with with 338 co-sponsors including by Rep. Yassamin Ansari, [D-AZ-3], Rep. Juan Ciscomani [R-AZ-6], Rep. Abraham Hamadeh, [R-AZ-8], Rep. David Schweikert [R-AZ-1], Rep. Greg Stanton, Greg [D-AZ-4].
A Grass Roots Movement
Getting this lifesaving legislation across the finish line was supported by coalition of advocacy organizations and individuals who worked together for years. They signed letters, contacted their elected leaders, and travelled to Washington, D.C. to share their stories.
They made a difference!