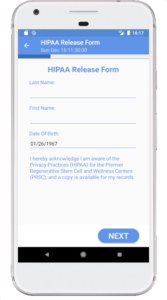
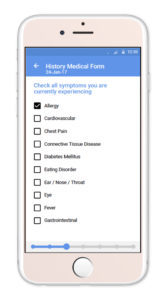
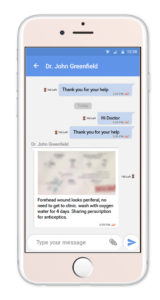
Starting April 1st, 2020 through July 1st, 2020, IMNA is offering free access to the TrialME platform for all teams researching COVID-19 and other life debilitating diseases.
TrialME offers sites a seamless shift to a hybrid structure that follows the FDA’s new guidelines during COVID-19 pandemic; the platform monitors, engages, and communicates with patients from the comfort and safety of their home.
 SCOTTSDALE, Ariz., – (April 6, 2020) – During the COVID-19 outbreak, IMNA Solutions is helping sites shift a number of on-site processes to a secure remote environment; ultimately, allowing for the continued operation of ongoing trials by addressing emerging real-world operational challenges, information gaps and providing an alternative means of participation for patients, who are, hesitant or unable to continue their treatment on-site. TrialME offers a comprehensive telemedicine program that is designed according to FDA’s newly issued guidelines for industry, investigators, and institutional review boards that are conducting clinical trials during the novel coronavirus (COVID-19) pandemic. TrialME enables investigators to engage with patients from the comfort of their homes.
SCOTTSDALE, Ariz., – (April 6, 2020) – During the COVID-19 outbreak, IMNA Solutions is helping sites shift a number of on-site processes to a secure remote environment; ultimately, allowing for the continued operation of ongoing trials by addressing emerging real-world operational challenges, information gaps and providing an alternative means of participation for patients, who are, hesitant or unable to continue their treatment on-site. TrialME offers a comprehensive telemedicine program that is designed according to FDA’s newly issued guidelines for industry, investigators, and institutional review boards that are conducting clinical trials during the novel coronavirus (COVID-19) pandemic. TrialME enables investigators to engage with patients from the comfort of their homes.
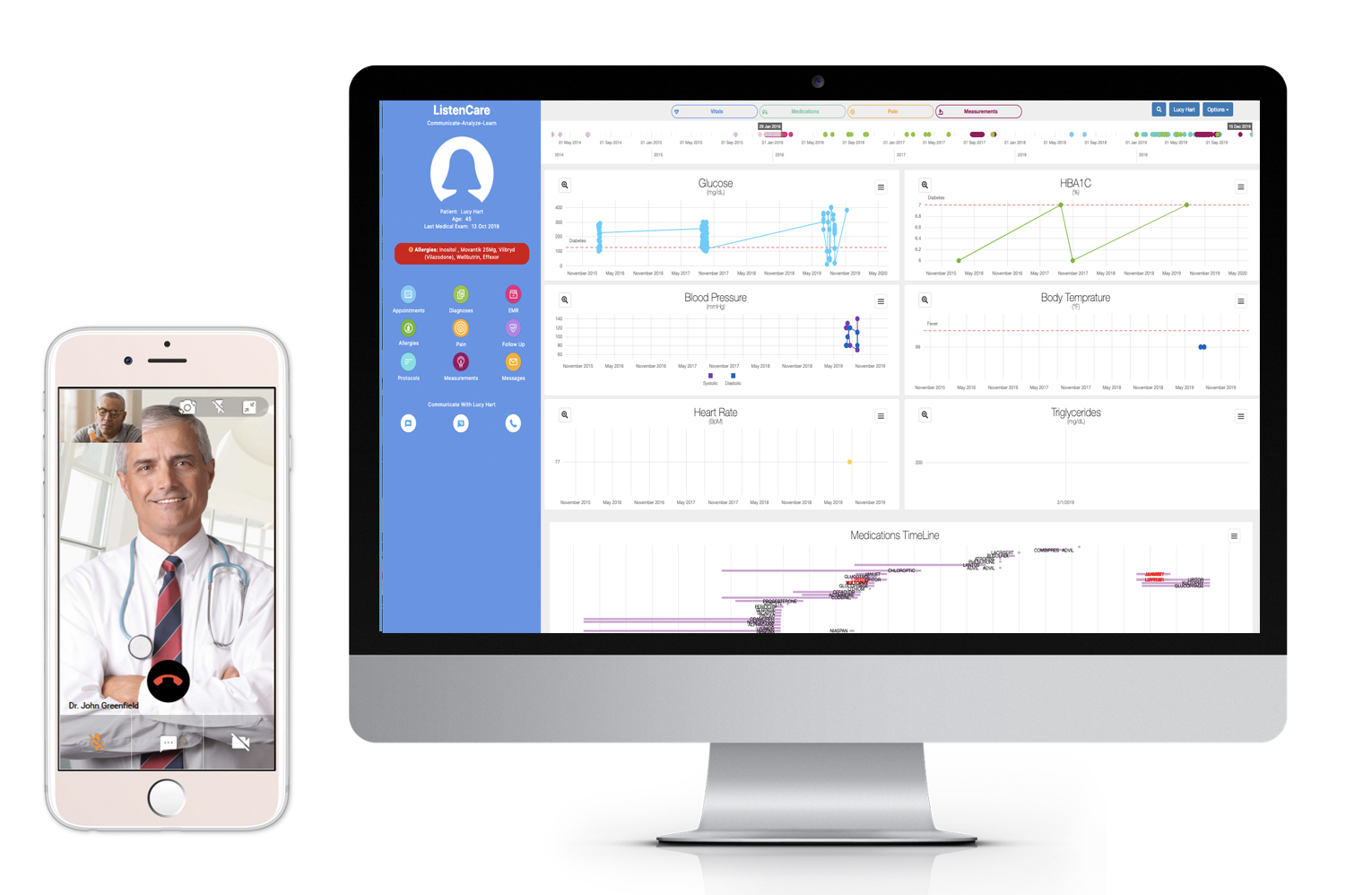
Among the unique core features of TrialME are the remote-monitoring capabilities, such as: patient reported outcomes, symptoms questionnaires, vitals and physiological trackers, real-time alerts, secured file sharing, video and talk consultation, and messaging. These core features provide risk-mitigation strategies as part of an imperative solution to protect the safety and well-being of participants. In supporting the ongoing collection and secure processing of patient data, TrialME provides a high-level of data accuracy and integrity that is critical for a trial’s success.
“Research studies are critical to improving COVID-19 and chronic care and, for patients with advanced disease, clinical trials are often their standard of care. The world is shifting and during this current crisis, we are proud to service and support the bioscience and pharma community with their need for Remote Patient Monitoring and Management Technology. Our TrialME platform helps keep clinical trials open and reduces the disruption of drug development. It is our mission to help trials stay on track and ensure the safety of trial participants, maintain compliance with good clinical practice, and minimize the risks to trial integrity during these challenging times,” says Israel Haikin, MBA, Founder and CEO of IMNA Solutions.
The COVID-19 program was developed according to the latest information available from official health authorities such as Center for Disease Control (CDC), World Health Organization (WHO), and U.S. Food and Drug Administration (FDA) with ironclad security (HIPAA, ISO-27001, ISO-27799, GDPR, 21 CFR part 11).
TrialME interacts with clinical trial participants throughout the trial’s lifecycle; enabling personalized, high-quality patient care while increasing operational efficiency. The TrialME platform uses telemedicine to ensure continuous monitoring, minimizes gaps in patient relations and increases retention.
About IMNA Solutions
IMNA Solutions, founded in 2016, is a software development company that has created a patented, highly secured data collection and patient management platform. IMNA takes a patient-centric approach to managing medical conditions for healthcare providers and researchers who are seeking to improve quality and level of care while reducing administrative tasks and costs. IMNA improves the process of data collection and reduces participant abandonment rate in clinical trials by facilitating the ultimate handshake between research teams and their patients.
TrialME interacts with clinical trial participants throughout the trial’s lifecycle; enabling personalized, high-quality patient care while increasing operational efficiency. The TrialME platform uses telemedicine to ensure continuous monitoring, minimizes gaps in patient relations and increases retention.
Media Contact:
Meirav Naor-Weinstock, Co-Founder and Chief of Business Development
IMNA Solutions
Meirav.naor@imnasol.com (646) 402-2408
https://www.linkedin.com/company/imna-solutions