Daniel O’Day – October 08, 2020
With today’s publication of new data on remdesivir in the New England Journal of Medicine, we have the clearest picture yet of the medicine’s impact on COVID-19. Over the past months, we had already generated a wealth of clinical data on the benefits of remdesivir, providing much-needed hope to patients and healthcare providers worldwide. On the basis of this data, remdesivir has been approved or authorized for temporary use to treat COVID-19 in more than 50 countries worldwide. Today’s peer-reviewed data come from the gold standard randomized, double-blind, placebo-controlled Phase 3 study led by the U.S National Institute of Allergy and Infectious Diseases (NIAID). They provide new information that expands on the benefits of remdesivir and offer greater hope than ever in the fight against COVID-19.
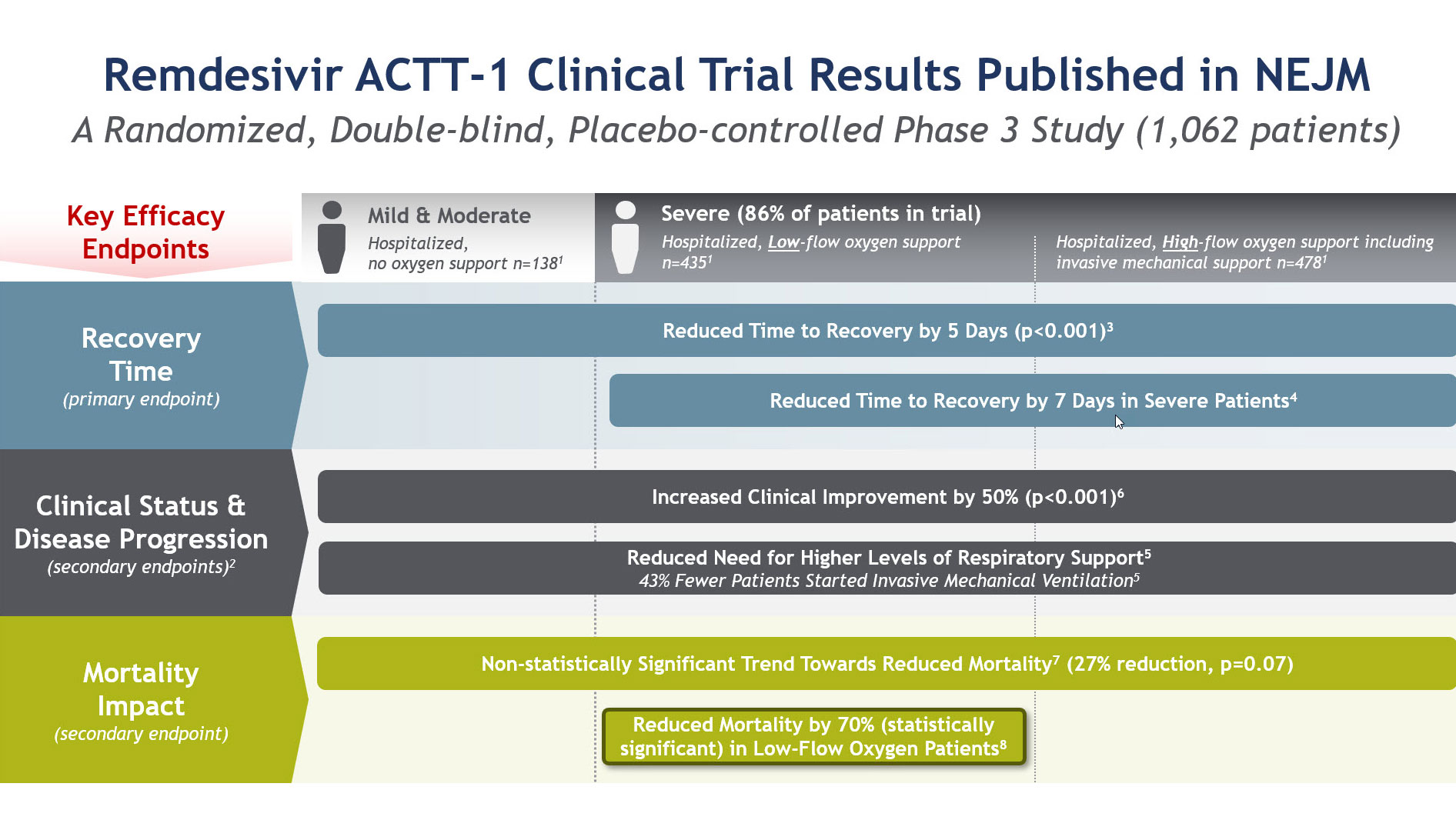
 The preliminary results from the global NIAID study were published in May, but now we have the final dataset covering approximately 1060 hospitalized patients worldwide. These data tell us three things.
The preliminary results from the global NIAID study were published in May, but now we have the final dataset covering approximately 1060 hospitalized patients worldwide. These data tell us three things.
The first is that hospitalized patients receiving remdesivir recovered five days faster on average, and in patients with severe disease, seven days faster. These severely ill patients made up 85% of the total study population. The second key takeaway is that remdesivir reduced the likelihood of patients progressing to more severe stages of the disease where they would require new or additional oxygen support. And thirdly, in the largest group of patients in the study, those on low-flow oxygen, there was a significant reduction in mortality in a post-hoc analysis. In the overall population, the results showed a trend in mortality reduction. Each of these study outcomes is of critical importance to managing the pandemic and informing decisions made by healthcare workers on behalf of their patients.
For patients who are hospitalized with COVID-19, the importance of speeding up recovery by five to seven days cannot be underestimated. Aside from the physical challenges, every day with the disease brings an emotional toll on patients and their families dealing with the separation and the worry. Some physicians have also raised the point that the longer a patient is in hospital, the greater the risk of getting a secondary infection. In the group of patients that required supplemental oxygen, those receiving remdesivir plus standard of care recovered one week faster than those treated with placebo plus standard of care. This represents a significant benefit in a disease where every day counts.
The latest data also include new results on reducing disease progression. In the group of patients receiving remdesivir, fewer progressed to the need for supplementary oxygen or higher levels of respiratory support, such as mechanical ventilation. Remdesivir works by reducing the ability of the virus to replicate in the body and ideally, we want to stop the replication as early as possible in the course of the disease. We know that for patients on a ventilator, the chances of survival are lower. By reducing disease progression, remdesivir may keep patients from entering this critical stage of illness.
The benefits of remdesivir in leading to faster recovery and a reduction in disease progression were seen broadly in the overall NIAID study results. In addition to the direct impact on patients, these outcomes represent clear benefit and value to healthcare systems. Remdesivir could help to lower the use of healthcare resources and reduce the number of days that patients are in the hospital.
The question of whether remdesivir provides a mortality benefit is one we have been looking forward to answering with peer-reviewed, placebo-controlled clinical data. It is important to note that the study enrolled a broad range of patients so there was significant heterogeneity in disease severity at baseline. Although the prespecified analysis of mortality in the total population showed only a numerical trend toward a reduction, there are additional findings on mortality in this study that provide valuable new information. In a post-hoc analysis of mortality rates for patients receiving low-flow oxygen, there were 70% fewer deaths among patients receiving remdesivir compared with the placebo group. Patients on low-flow oxygen made up approximately 40% of the overall patient population. This information is critically important for understanding remdesivir’s potential impact on mortality and maximizing the benefit to patients.
Today’s new results add to the totality of evidence on remdesivir that has been generated across three randomized controlled Phase 3 studies. All of us at Gilead are grateful that, along with the trial investigators and thousands of patients who have taken part in clinical trials, we have generated consistent data to help inform treatment decisions that will be taken on behalf of many more patients with COVID-19 in the future.
Meeting global demand
At the same time as receiving this additional evidence on the benefits of remdesivir, we have been able to significantly increase supplies. In the United States, we have enough remdesivir on-hand to treat all hospitalized patients, even in the event of a future spike in cases. Globally, we expect to meet demand this month, enabling the purchase of Veklury both to treat current hospitalized patients and to support national stockpiling. Earlier today, Gilead announced an agreement with the European Commission that enables 37 participating countries in the EU and the European Economic Area (EEA), as well as the UK, to purchase remdesivir for real-time demand and stockpiling needs, coordinated by the European Commission. The agreement covers purchases of remdesivir over the next six months with the option to extend further. In addition, Gilead’s voluntary licensing partners are currently supplying generic remdesivir in more than 40 countries, and their patient reach continues to grow.
It is remarkable that we are in this position today, given that remdesivir is such a complex medicine to manufacture with a typical lead time of 9-12 months. Gilead has left no stone unturned in the efforts to bring us to this point. We invested in ramping up manufacturing long before we knew whether remdesivir would work, we brought on more than 40 additional manufacturing partners and our teams worked day and night to find ways to shorten the lead time, without compromising on safety or rigor. I am grateful to the many Gilead employees, contract organizations and partner companies who worked so hard to make this happen, overcoming many challenges along the way.
Our journey with remdesivir has entered a new phase, with the potential to make an even greater impact on the pandemic. We have rich new data to show how it might best be used to benefit patients and, with the increase in supply, many more patients can now access remdesivir worldwide. At the same time, we continue to explore its potential. Ongoing studies include intravenous treatment in the outpatient setting and an inhaled solution that could potentially be taken earlier in the course of the disease. We are also looking forward to the results of combination studies where remdesivir is the backbone treatment, to which other therapies with complementary mechanisms of action may be added. As we move forward with remdesivir, we will continue to be guided by the most robust clinical data at each step of the way, in the hope that science will continue to deliver in the fight against COVID-19.
- Sub-group “n” values add up to 1,051 instead of 1,062 because 11 Patients did not have a severity baseline score recorded
- Clinical Status was a pre-specified key secondary endpoint, and disease progression was a pre-specified secondary endpoint
- From 15 days to 10 days, an increased recovery rate of 29% compared with placebo; rate ratio for recovery 1.29; 95% CI 1.12-1.49; p<0.001
- From 18 days to 11 days; rate ratio 1.31; 95% CI 1.12-1.52; severe disease was defined as requiring mechanical ventilation, requiring oxygen, a SpO2 ≤ 94% on room air, or tachypnea (respiratory rate ≥24 breaths/min)
- Incidence of new use of oxygen (36% remdesivir vs. 44% in placebo), new high-flow oxygen (17% remdesivir vs. 24% placebo), and new mechanical ventilation or ECMO (13% remdesivir vs. 23% placebo) were all lower in those patients treated with remdesivir compared with placebo
- Compared with placebo, remdesivir treatment effect was maintained at Day 15 through Day 29 (OR: 1.50; 95% CI 1.2-1.9; P<0.001)
- 11.4% mortality in patients treated with remdesivir vs. 15.2% with placebo at Day 29; HR 0.73 [95% CI 0.52-1.03]; p=0.07
- Post-hoc sub-group analysis performed across all sub-groups (not accounting for multiplicity); 70% reduction in mortality compared with placebo (HR 0.30 [95% CI 0.14-0.64]); remdesivir treatment group n=232, placebo group n=203
About Veklury
Veklury (remdesivir) is an investigational nucleotide analog invented by Gilead, building on more than a decade of the company’s antiviral research. Veklury has broad-spectrum antiviral activity both in vitro and in vivo in animal models against multiple emerging viral pathogens, including Ebola, SARS, Marburg, MERS and SARS-CoV-2, the virus that causes COVID-19.
Multiple ongoing international Phase 3 clinical trials are evaluating the safety and efficacy of Veklury for the treatment of COVID-19. Based on available data from these studies, Veklury has been approved or authorized for temporary use as a COVID-19 treatment in approximately 50 countries worldwide. Additional ongoing international clinical trials continue to further evaluate the safety and efficacy of Veklury in different patient populations and formulations, and in combination with other therapies.
In the U.S., the U.S. Food and Drug Administration (FDA) granted Veklury an Emergency Use Authorization (EUA) for the treatment of hospitalized patients with COVID-19. This authorization is temporary and may be revoked, and does not take the place of the formal new drug application submission, review and approval process. Veklury has not been approved by the U.S. FDA for any use, and the safety and efficacy has not been established. For information about the authorized use of Veklury and mandatory requirements of the EUA in the U.S., please review the Fact Sheets and FDA Letter of Authorization available at www.gilead.com/remdesivir.
Forward-Looking Statement
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors. Veklury is an investigational drug that has not been approved by the FDA for any use, and it is not yet known if Veklury is safe or effective for the treatment of COVID-19. There is the possibility of unfavorable results from ongoing and additional clinical trials involving Veklury and the possibility that Gilead and other parties may be unable to complete one or more of such trials in the currently anticipated timelines or at all. Further, it is possible that Gilead may make a strategic decision to discontinue development of Veklury or that FDA and other regulatory agencies may not approve Veklury, and any marketing approvals, if granted, may have significant limitations on its use. As a result, Veklury may never be successfully commercialized. In addition, there is also the risk that Gilead may be unable to effectively manage the global supply and distribution of Veklury. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. The reader is cautioned not to rely on these forward-looking statements. These and other risks are described in detail in Gilead’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2020, as filed with the U.S. Securities and Exchange Commission. All forward-looking statements are based on information currently available to Gilead, and Gilead assumes no obligation to update any such forward-looking statements.
Republished with permission: https://stories.gilead.com/articles/an-open-letter-from-our-chairman-and-ceo-oct-8